Columbia Engineers Develop New, Low-Cost Way to Capture Carbon
Researchers discover new reverse chemical reaction at nanoscale that enables efficient carbon capture
A recent study led by Xi Chen, associate professor of earth and environmental engineering at Columbia Engineering, and Klaus Lackner at Arizona State University, reports an unconventional reversible chemical reaction in a confined nanoenvironment. The discovery, a milestone in clarifying the scientific underpinnings of moisture-swing chemical reaction, is critical to understanding how to scrub CO2 from the Earth's atmosphere, and the researchers have already used it to capture CO2 more efficiently and at a much lower cost than other methods.
Water is the key player in this new study. The group found that reducing water quantities in nanoconfinement could promote CO32- (carbonate) ions to hydrolyze H2O into a larger amount of OH- (hydroxide) ions. This discovery also led the team to find a new nanostructured CO2 sorbent (a material used to absorb or adsorb liquids or gases) that also binds CO2 spontaneously in ambient air when the surrounding is dry, while releasing it when exposed to moisture. The work was published in Angewandte Chemie in February 2016.
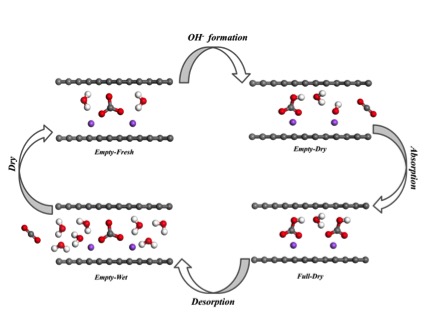
Reaction pathway of CO2 absorbption / desorption on
nanostructural absorbent
“Water confined in nanoscopic pores is essential in determining the energetics of many chemical, physical, biological, and environmental systems,” says Chen. “Our finding sheds light on a vast number of chemical processes in nanoconfinement while also giving rise to a wide array of potential applications. For instance, we can convert this new efficient sorbent from absorption to desorption simply by using water, which is readily available and at very low-cost. Current sorbent materials consume a great deal of energy, so our discovery could lead to cheaper and more efficient energy conservation absorbents. And if we can achieve negative carbon emission standards, then we will have invented a nanomaterial solution to a critical global challenge.”
Finding an efficient absorbent has long been a challenge for most absorption and desorption processes. A successful CO2 absorbent must have fast reaction kinetics (the rate of chemical processes), be low in cost, and be able to regenerate with a low energy barrier to complete the whole CO2 capture-release cycle. Chen notes that, to his knowledge, all previous CO2 absorbents have required a large energy barrier to regenerate and are thus not very efficient, consuming more extra energy to regenerate. The mechanism of the moisture-swing chemical reaction in nanopores will lead to new classes of sorbents driven by water: evaporation in ambient air through solar energy drives the sorbent to absorb CO2 as it dries, and hydration releases CO2 when wet.
“With water as the trigger, our energy cost of the whole CO2 capture cycle is very small,” Chen adds, “and that makes grand-scale application very promising for the first time.”
Chen, whose research is focused on the mechanics of nanoporous materials, has long been interested in studying fundamental interactions between water and ions in a confined space. When confined to nanopores, the hydrogen bonding of water and ions changes and this affects both the physical structure and dynamics of water molecules and the chemical energy transfer through the formation of highly structured water complexes.
“Water is the most magical substance in the world—it produces life,” says Chen, who worked on the study with Klaus S. Lackner, formerly at Columbia Engineering and now director of the Center for Negative Carbon Emissions and a professor at Arizona State University. “Its hydrogen bond is incredibly strong—except, as we discovered, when you have a very small environment with very few molecules. Then everything changes and we were able to actually reverse chemical reactions when the number of water molecules fell below about 10.”
Chen’s team ran experiments to control the humidity in the nanoporous material and found that the free energy of CO32- hydrolysis in nanopores is reduced with a decrease of water availability. This process promotes the formation of OH-, which has a high affinity to CO2. They also found that this humidity-driven sorption effect is not limited to carbonate/bicarbonate but is also extendable to a series of ions and thus the study opens a new approach to gas separation technology.
“This is an outstanding work, ” comments Agustin J. Colussi, senior scientist at the Linde Center for Global Environmental Science, California Institute of Technology, who was not involved in the study. “It reports convincing experiments, provides a novel explanation for counterintuitive results, and thereby opens the vast scope of new chemistry in nanoconfined water.”
Chen and his team, which includes his PhD students Xiaoyang Shi (the study’s lead author) and Hang Xiao (second author), plan to explore the influences of a range of parameters, including pore size, spacing of ions, and surface hydrophobicity, on his water-driven CO2 capture absorbent, to design a more efficient direct air capture CO2 system.
This research was partially funded by the Office of Naval Research (ONR) and by the Center for Negative Carbon Emissions at ASU.