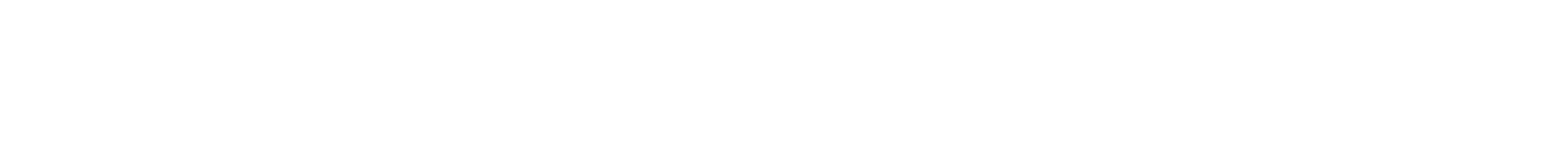Prof. Vunjak-Novakovic Wins $6.3M NIH Grant to Design Tissue Chip to Evaluate Drugs
The National Institutes of Health (NIH) has just announced major funding for the next three-year phase of its Tissue Chip for Drug Screening program, aimed at improving ways of predicting drug safety and effectiveness, and a team led by Gordana Vunjak-Novakovic, Mikati Foundation Professor of Biomedical Engineering and professor of medical sciences, has been awarded an additional $6.3 million of funding over three years, with $3.5M going to Columbia Engineering. The project consortium includes three of the leading laboratories in the country: Sangeeta Bhatia (MIT), Christopher Chen (Boston University in collaboration with Harvard’s Wyss Institute for Biologically Inspired Engineering), and Karen Hirschi (Yale University).
Striated structure of human cardiac muscle used to study drugs in the HeLiVa platform
Today, the estimated cost of bringing a new drug to clinics exceeds $1 billion. Still, some 80 percent of candidate drugs fail in human clinical trials because they are found to be unsafe or ineffective, according to the NIH, despite promising preclinical studies in animal and cell models. The majority of drug recalls in the past 40 years have been due to cardiotoxicity (19% of withdrawals), hepatotoxicity (26% of withdrawals), or unpredicted adverse effects of drug interactions. Drug toxicities that pass through preclinical and clinical studies are particularly highly costly. This situation is largely due to poor predictions of drug responses in the human body by both cell culture and experimental animals, and has motivated the development of tissue chips for drug screening.
Vunjak-Novakovic and her team are developing an integrated heart-liver-vascular model system (nicknamed HeLiVa chip) that mimics the function of the human body and can be used to evaluate therapeutic drugs. The system can also be personalized to model specific genetic and disease states to test drugs for their effectiveness and toxicity in the heart or liver.
“This is a very important program and an exciting collaboration between biology and engineering,” says Vunjak-Novakovic.
“The development of tissue chips has the potential to transform preclinical testing of candidate treatments, providing valuable tools for biomedical research,” adds NIH Director Francis S. Collins, MD, PhD.
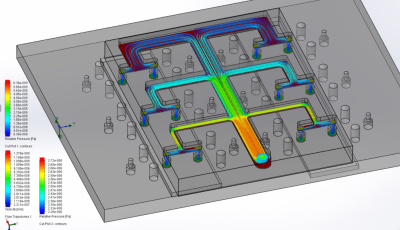
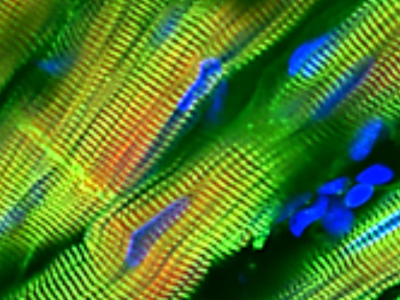
Distribution of fluid flow in the HeLiVa platform (six chambers are shown)
For their part, Vunjak-Novakovic’s team is working on combining 3-D human tissue chips into an integrated platform that can mimic the complex functions of the human body. She points out that tissue engineering is now becoming increasingly successful in more authentically representing the actual in vivo milieu of development, regeneration, and disease. “While it is unreasonable to expect bioengineered models to exactly match native tissues, they can recapitulate certain physiological functions and be used to investigate drug efficacy, safety, and mode of action,” she explains. “Instead of attempting to achieve the complexity of an organ, our team seeks to identify the simplest tissue unit allowing predictive studies of human physiology. Such units, which measure just one or few millimeters, replicate the tissue-specific architectures and the most relevant organ functions.”
Her group is deriving multiple “organs on chips” starting from a single population of the patient’s stem cells. This approach allows drug screening and disease modeling in a patient-specific manner. Modular microtissue platforms can capture the salient features of multiple human tissues connected by the vascular network. These microphysiological platforms can be further interfaced with functional imaging, to enable real-time monitoring of physiological responses at molecular, cellular, and organ levels. Such interactive microtissue systems offer enormous complexity and diversity of responses to drugs and disease that can be modeled (e.g., cardiotoxicity of drugs metabolized by liver, inflammation, hypoxia due to the lack of blood supply).
“After the first two years of our research, we are very excited to continue our work,” says Vunjak-Novakovic. “We are making great progress in bioengineering human microtissues that represent a working heart muscle, a metabolizing liver, and non-thrombogenic vasculature—these will help us face the major challenges ahead in this complex and demanding project.”
The NIH program, which is led by the National Center for Advancing Translational Sciences (NCATS), will support 11 institutions at $17 million in 2014. NIH has committed nearly $76 million over the course of the five-year program, which was launched in 2012.