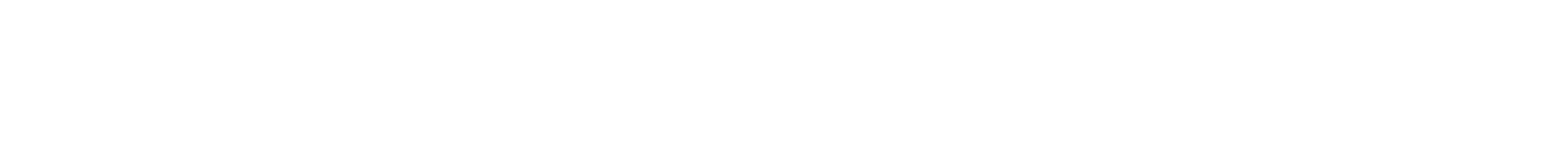Transformers
Researchers at the Columbia Electrochemical Energy Center chart the future of clean energy storage.
More than 200 years since their invention, batteries have become ubiquitous, yet many aspects about them remain poorly understood. For most, the matter of portable energy storage is purely a functional issue: if it works, we’re happy. We never question why they are shaped like cylinders or rectangular solids, or what happens inside them.
For decades, not even engineers gave them much thought.

THE BROOKHAVEN CONNECTION
Under lab conditions, researchers can make remarkable progress studying battery systems and fuel cell catalysts. But to properly assess how these platforms behave while operating in the real world requires something else altogether—specifically, something called a synchrotron.
“When I was graduating, I never would have imagined that anyone would have cared about batteries except for a few of my friends,” notes Professor Alan C. West, an expert in electrochemical systems.
But then climate change changed the game. Our greatest hope for charting a sustainable future lies in renewable energy—but unlike fossil-fueled power plants that produce electricity on demand, renewables like wind, solar, and water require storage solutions on an unprecedented scale. The sun doesn’t shine continuously every day or as strong in winter, and the wind doesn’t blow as hard in summer. But human society needs consistent electricity all year long. At the same time, more people are crowding into urban centers, necessitating stepped-up efforts toward developing green transportation and buildings.
The need is urgent. Global commitments— framed by the International Energy Agency and the Paris Agreement—established deadlines for reduction in fossil fuel emissions and progress in incorporating renewable energy sources into the national grids, but efforts are behind schedule in almost every region.
In the United States, cities and states will have an increasingly critical role to play. In Columbia University’s home state of New York, the Clean Energy Standard mandates reducing greenhouse gases by 40 percent by 2030, with 50 percent of the state’s electricity generated through renewable energy sources; current levels of the latter hover around 25 percent. New York City is aiming to cut greenhouse gas emissions by 80 percent of 2005 levels by 2050 through reductions in the power, transportation, waste, and building sectors.
But even as solar, wind, and water technologies have made great strides—and dramatically decreased in cost—their potential cannot be realized without leaps in battery storage and fuel cell technology, as well as similar drops in their price points.
The Columbia Electrochemical Energy Center (CEEC) has launched with those goals in mind. Its raison d’être: to connect the dots between discovery in the basic science of storage and the engineering of practical solutions for all applications. Its goals: to push the frontiers of electrochemical science and technologies needed for the widespread adoption of renewable electricity and to advance sustainable production of chemicals and fuels powered by renewable electricity. Ultimately, it will tackle the biggest challenges facing widespread adoption of electric transportation, clean urban buildings, grid-level storage of renewables, and the use of battery systems to equalize energy access.

CEEC codirector Alan West (Photo by Kyle Parsons)
The breadth of our talent and resources is enabling us to rapidly evaluate concepts and prototype material.
The new center takes a whole-systems approach, connecting engineers having technical expertise and interests ranging from atoms to grid systems, as well as scientists and policy makers. “These are big, meaty problems,” says Dan Steingart, associate professor of earth and environmental engineering, who joins the center from Princeton University this spring as CEEC’s codirector alongside West. “It would be hard to work on them without a group of people this good.”
That diversity of talent is one of the center’s greatest strengths. “We took a look at the array of resources each of our departments had built up and realized that we span the scale continuum from materials to systems,” says West. “The potential we can tap by having them collaborate on the storage challenge is enormous. And we’re addressing this complex problem through a humanistic lens that will ensure scalable, economical, just, and human-centered solutions through fundamental breakthroughs and implementation.”
The scope of the challenge is vast, with no silver bullet in sight. Electric vehicles, green buildings, national grids, and the more than a billion people without access to electricity— these each present unique issues. Faculty are exploring a wide range of storage technologies, from batteries to fuel cells, and testing them via experiment and simulation within the University, while also collecting data in the field, under real-world conditions. For instance, the center will establish a testbed to study how battery systems interact with multiple other efficient technologies, including thermal storage, along with ancillary controllers and software. The potential for a new building on the University’s Manhattanville campus would allow Columbia Engineering to create a living laboratory for clean energy.
“We are agnostic as to which technology we pursue for any one application,” says West. “The breadth of our talent and resources is enabling us to rapidly evaluate concepts and prototype material.”
Connecting atoms and architectures to systems and grids, CEEC’s scientists are advancing their individual expertise while creating a sum much larger than its parts.
Nanoparticles are 500 to 100,000 times thinner than a human hair, but when it comes to batteries, features on such a minute scale pull many of the strings. “Slight changes to either the composition or the atomic structure of a material can have a profound impact on the material’s properties,” says Alexander Urban, an assistant professor of chemical engineering who joins the center in January by way of the University of St. Andrews and University of California, Berkeley. Urban’s goal is to find new materials that will reduce degradation at the electrode surfaces where they interact with the electrolyte—a limiting factor for the lifetime of lithium-ion batteries and solid-oxide fuel and electrolyzer cells (SOCs). Understanding the atomic-scale mechanisms that govern such degradation reactions, Urban says, will lead to the discovery of resistant materials or coatings to protect the electrode. By employing atomic-scale and electronic-structure computations to create simulations, he investigates ceramics for SOCs and alkali-ion batteries (especially the lithium-ion variety). The electrodes of both these technologies are typically made of oxides with complex defect chemistry and electronic properties that affect the device performance, and computations are the ideal tool to understand their inner workings.
“On the one hand,” Urban says, “it can be a frustrating task to unravel the structure-composition-property relationships in energy materials. On the other hand, the large chemical and structural space that we draw from offers great opportunities for the design and discovery of improved materials.”

Alexander Urban (Courtesy of Alexander Urban)

Lauren Marbella (Photo by Kyle Parsons)
Improving materials is also the goal of Assistant Professor of Chemical Engineering Lauren Marbella, fresh from postdoctoral studies at the University of Cambridge. She studies materials on small length scales as well, working over multiple orders of magnitude (from nanometers to micrometers) to reimagine ways to formulate and evaluate energy storage systems. By developing nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI) techniques capable of capturing processes at the atomic level, she can observe phenomena as fine-grained as the diffusion of lithium ions. Improving the visualization of lithium (and other key elements in battery chemistry, such as sodium, carbon, oxygen, and fluorine) in situ is crucial to understanding how the act of moving energy through a storage device can alter its very structure.
“We don’t understand to a very basic degree what’s happening in battery materials as they charge and discharge,” she says. “For instance, once a crystalline structure becomes amorphous during lithiation, it may never regain its crystallinity. That changes how it will behave from there on out.”
But observing a static battery cycle only reveals part of the picture. Marbella is also building a mechanical system capable of bending and stretching batteries while inside an MRI machine. That kind of real-world stress produces damage at every level of a storage platform, leading Marbella to question whether a whole new class of materials might be needed. “Lots of things in nature go through large-scale mechanical and structural changes; fibrous proteins allow them to expand and contract safely. Could we create biologically inspired materials that allow energy storage platforms to do the same?” she wonders. “In the case of all solid-state batteries, you have hard materials butting up against each other. Could we create ‘joints’ at those sites, the way nature does?” To find out, Marbella is collaborating with colleagues in chemistry, biology, and chemical engineering.
The opportunity to work with like-minded researchers focusing on electrochemical energy up and down the scale continuum is key for knowledge discovery, she says, and a significant factor in her decision to join Columbia Engineering.
Lots of things in nature go through large-scale mechanical and structural changes. . . . Could we create biologically inspired materials that allow energy storage platforms to do the same?
SRS imaging shows the ionic concentration distribution at lithium surface, ionic flux, and lithium deposition at the same time. (Courtesy of Qian Cheng)
Yuan Yang (Photo by Kyle Parsons)
Batteries themselves are premised on smooth collaboration, between the anode—or negative electrode—on one end; the cathode—or positive electrode—at the other; and the electrolyte that ferries ions between them. Currently, limitations on capacity, charging speeds, and power levels are impeding battery adoption for a number of applications, not the least of which is electric transportation. Theoretically, switching to lithium metal electrodes is one of the best ways to improve communication and transcend those limitations. “Lithium metal has the highest theoretical volumetric capacity and the most negative potential of any element at room temperature,” says Yuan Yang, assistant professor of materials science and engineering. There’s just one problem: “It is also the most difficult material to manipulate reversibly.”
One reason is lithium’s propensity to form uneven or rough surfaces, with irregularities amplifying as the battery is charged and discharged. The growing surface roughness can lead to dendrites, which result in unpredictable behaviors that, at best, reduce lifetime and, at worst, create critical risks, such as fires. Why and how these irregularities grow has long bedeviled researchers, partly because there was no way to study the process as it occurred. “I realized no one was looking at liquid ion movement, and I thought, why?” says Yang. “It was because there were no existing materials science tools that were fast enough to capture it.”
Yang, who studies fundamental processes in battery reactions, turned to stimulated Raman scattering (SRS) microscopy—a high-speed technique developed by Columbia Chemistry Professor Wei Min—to map how ion movement in the battery’s electrolyte adversely affects reactions at the electrode.
“We witnessed how ions move in a liquid electrolyte, something that no one was able to observe in the past,” Yang says. “SRS was developed for life sciences. Few in materials science even know about it.”
What they confirmed were three stages of dendrite growth, and a strategy for mitigating it during two phases—a finding with wide-reaching implications. “Fuel cells also rely on ion transport,” notes Yang.
The fuel cell operates by converting chemical fuels into electricity. Such fuels have the virtue of being highly versatile—while hydrogen-powered vehicles are perhaps the best-known application of the technology, a wide range of industrial, commercial, and residential uses are possible. For instance, hydrogen’s tremendous storage potential—in both capacity and duration—makes it especially well-suited to tackle large-scale challenges, such as grid-level storage. Unfortunately, there’s no economical way to capitalize on this technical advantage until researchers can substantially reduce conversion costs associated with producing hydrogen fuel.
Chemical Engineering Professor Jingguang Chen—an expert in hydrogen chemistry as well as surfaces and the electrochemical reactions that occur there—sees cheaper catalysts as a big part of the solution. “Many systems use very expensive precious metals, such as platinum and iridium,” he explained, “and there may not be adequate supply in the world.” Chen’s group is actively investigating nonprecious metal electrocatalysts for water electrolysis and fuel cell systems. At the same time, he is employing computational and experimental methods to find a way to make the current catalyst materials more efficient. He maintains part of his research group at Brookhaven National Laboratory, using its synchrotron and computational facilities for monitoring nano- and molecular-level behavior.

Light-generated hydrogen bubbles evolving from the surface of a photoelectrochemical solar cell in the Solar Fuels Engineering Lab, headed by Dan Esposito. (Image courtesy of PhD candidate Anna Dorfi and high school students Jhaelle Payne and Ellie Prickett)

Dan Esposito (Photo by John Abbott)
If researchers can find a way to produce these chemical fuels using CO2-free solar energy, carbon emissions would be eliminated from the process. Two such promising approaches there are photovoltaic (PV)- and photoelectrochemical (PEC)-electrolysis cells, which use sunlight to convert low-energy reactant molecules such as water or CO2 into energy-dense fuels such as hydrogen or methanol.
Dan Esposito, assistant professor of chemical engineering, heads a group designing novel materials and device concepts for solar fuels production using PV-electrolyzers and PECs. Their research illuminates how the optical, electronic, and catalytic properties of catalytic materials influence efficient chemical conversion processes at the electrode/electrolyte interface. With in situ spectroscopic and imaging tools, his team is studying how materials and devices perform while operating in electrochemical environments that mimic real-world conditions.
In a recent study, Esposito’s group “glued” platinum nanoparticles to electrode surfaces with a nanoscale layer of silicon oxide, in essence encapsulating them, and observed how this membrane controlled which reactants in the electrolyte gained access to the electrode. This architecture offered a promising approach to enhancing electrode stability while providing resistance to contamination from impurities and enabling a means of controlling competing reaction pathways.
“The challenges in demonstrating solar fuel technologies that are stable, efficient, and cost-effective span many length scales and range from fundamental to applied in nature,” Esposito says. “But we are optimistic that we have a team of researchers at CEEC with the talent and tools to help move this technology forward.”
Alan West’s electrochemical engineering lab works on optimizing designs using a variety of materials that can be incorporated into either batteries or fuel cells, work that spans the continuum scale and connects his lab to researchers across the center. “Basically, we study how quickly we can move the materials through the electrolyte and electrode structures without losses,” West says. For instance, he has explored how magnetite, or iron oxide, a prospective electrode material, behaves in battery systems along the scale from the molecular to the meso-, especially in regard to inefficiencies that could lead to undesirable amounts of heat if deployed in a large system.
Supplied with the data that his CEEC colleagues generate for various materials and architectures, West and his group build mathematical models that characterize and simulate thousands of conditions in order to understand how to modify a material’s architecture to optimize performance—a particularly challenging, but essential, exercise due to the highly complex and nonlinear nature of battery systems, which never actually operate in an ideal fashion.
One critical aim of such an iterative modeling process is to find a way to boost the transport speed of lithium-ion atoms, a significant gating factor for improving battery performance. Ultimately, this will lead to electric cars that can be charged in 15 minutes—a 48-fold increase in speed over charging stations in wide use today, and five times faster than a Tesla Supercharging station. “The real breakthrough will come at the nanoscale, and we need those breakthroughs to make real advances,” West notes.
When you stop saying you can’t let something happen, it opens up certain pathways.
“I’m the experimental counterpart to Alan,” says Dan Steingart, with a laugh. “Typically, there’s a gap between those who design materials and those who design the systems where they are applied—think of the difference between the ‘anatomy’ and ‘physiology’ of a battery, and how they can be mutually refined.”

Dan Steingart
In truth, living organisms are likely the only entities more complex than the humble battery. From an electrode’s atomic structure to a full battery pack, the interplay of disparate materials, electrochemical reactions, systemic calibrations, and unintended consequences is maddeningly intricate. “Making certain things better can make other things worse,” he says, meaning it takes more than a little intellectual judo to flip a battery’s negative properties into positive outcomes. Take those troubling short circuits. Knowing that, in many situations, there isn’t a one-to-one correlation between a short and a fire, Steingart headed into his lab to investigate what kinds, or what level, of short-circuiting might actually be tolerable.
“When you stop saying you can’t let something happen, it opens up certain pathways,” he says. “It turns out that by creating a constantly short-circuiting battery, we created a battery that couldn’t die.”
The key is not just asking unconventional questions—it’s listening for unexpected opportunities, as with the million-dollar question that’s been stumping car manufacturers: “Why can’t we charge batteries quickly? Modeling-wise, Alan can show how to do it,” Steingart says. But to translate such advancements to the marketplace dramatically increases production costs—the battery is by far the most expensive part of an electric drive train, accounting for about 30 percent of total cost of the vehicle.
To solve that riddle, Steingart is embarking on a project to uncouple energy needs from power requirements in electric vehicles. “Temperature is one knob you can turn there,” he says, noting that warmer batteries can charge faster but colder ones suffer less self-discharge. Building on that insight, “now I could go to Yuan or Lauren and ask them to stabilize materials at certain temperatures, shifting the ‘Goldilocks’ balance on what is too hot and what is too cold,” he says.
The next step would be to provision batteries for power applications, such as plug-in hybrid and electric vehicles, as well as stationary grid storage. Matthias Preindl, assistant professor of electrical engineering, focuses on the design and control of power electronic and motor drive systems for these applications.
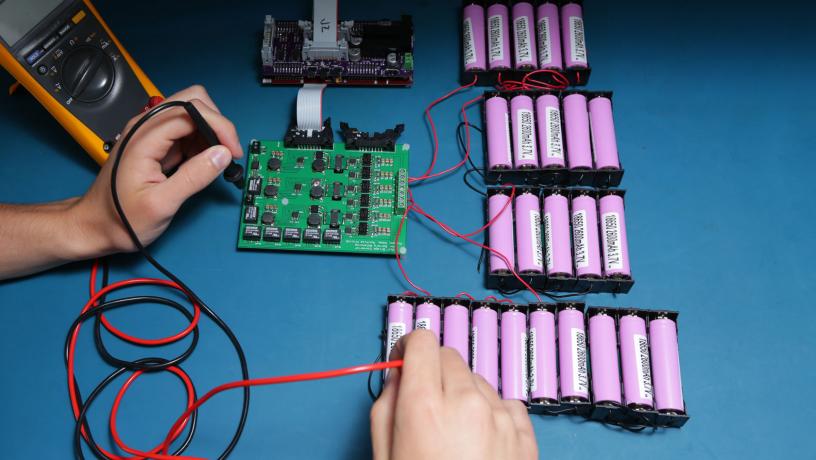
To meet power requirements here, battery cells must be connected serially—from a handful to thousands—in various configurations that optimize performance. Because individual cells behave inconsistently under real-world conditions—charge levels in cells drift apart due to effects both internal (manufacturing tolerances) or external (temperature variations across the pack)—optimization requires monitoring each cell in real time. To do that, Preindl uses machine learning and optimization-based estimation and control techniques to create models that are both sufficiently accurate and computationally inexpensive enough to account for thousands of cells simultaneously. By estimating each cell’s state-of-charge, as well as its potential deterioration, or so-called stateof-health, these models harmonize performance across the entire pack.
Conventional battery packs equalize stateof-charge between cells passively, through the addition of resistors. While inexpensive, this hampers performance. Preindl’s active balancing hardware, on the other hand, precisely moves charge from higher-energy cells to lower-energy ones by drawing on data. “Our integrated and modular technology effectively cuts the costs of active balancing hardware in half,” he says. “At the same time, the pack is no longer limited by the performance of the weakest cell, but can use all energy in the pack.”
This approach also extends pack life span. Inevitably, some cells in a series will deteriorate faster than others, subjecting the weakest cells to the highest stresses. Preindl’s technology addresses this problem in two ways. First, using stronger cells to support weaker ones reduces stress levels and slows deterioration rates. Second, because all cells operate at full capacity, vehicle range isn’t compromised by one or more weak elements.
For anyone in the developing world unconnected to the grid, or connected to an unreliable one, even storage at the level of a 12-volt car battery is life-transforming. Connecting battery packs to communal solar generators—creating in essence a local “mini-grid”—could thus transform entire villages. With support from Columbia’s Earth Institute, Mechanical Engineering Professor Vijay Modi leads a group developing a usage-based, card-enabled payment system being implemented in many countries across sub-Saharan Africa, in order to economically and efficiently facilitate these sorts of grids. Through use of a smart controller, their system optimizes energy management according to battery state and varying supply and demand, enabling families consuming differing amounts of electricity to draw power in turn.

Modi’s group is developing a usagebased, card-enabled payment system to facilitate local minigrids. The system optimizes energy management through a smart controller. (Photo Credit: Timothy Lee Photographers)

Vijay Modi (Photo Credit: Timothy Lee Photographers)
Local mini-grids have only been made practical, Modi says, by the steep drop in the price of solar and electronics over the last 15 years—a 10-fold decrease in some instances, with prices as low as three cents per unit in countries that combine strong sunlight with low labor costs. “This has kicked off a revolution. Providing electricity when you want it, when the sun is not shining, is the trillion-dollar question,” he says, adding that India alone is considering spending several hundred billion dollars over the next few years on solar and wind energy.
As director of the Quadracci Sustainable Engineering Laboratory, Modi has collected nearly a decade’s worth of data on how solar-powered mini-grids backed by batteries operate in hot, dusty conditions. That data is helping him and CEEC colleagues Yang, Steingart, West, and Preindl refine the controller algorithms for intelligent battery management systems. The ideal scenario will enable users to exploit any surplus electricity, reducing the need for larger-scale storage. And not just in the developing world. Any homeowner anywhere in any country equipped with such a system could put that surplus to work. The possibilities are staggering.
“If we can cut the cost of batteries in half, and make them last twice as long, we are poised to change the lives of the bottom billion in a way few other technologies have,” Modi says.
Welcome to the Columbia Electrochemical Energy Center